Introduction
The Electronic Clinical Outcome Assessment (eCOA) Market is transforming the landscape of clinical research by digitizing patient data collection and enhancing the accuracy of clinical outcomes. eCOA systems leverage electronic devices such as smartphones, tablets, and computers to record patient-reported outcomes (PROs), clinician-reported outcomes (ClinROs), and observer-reported outcomes (ObsROs). By replacing traditional paper-based methods, eCOA platforms improve data quality, compliance, and efficiency in clinical trials. The market’s growth is being driven by the pharmaceutical and biotechnology industries’ increasing adoption of digital tools to streamline trials, comply with regulatory standards, and accelerate drug development timelines.
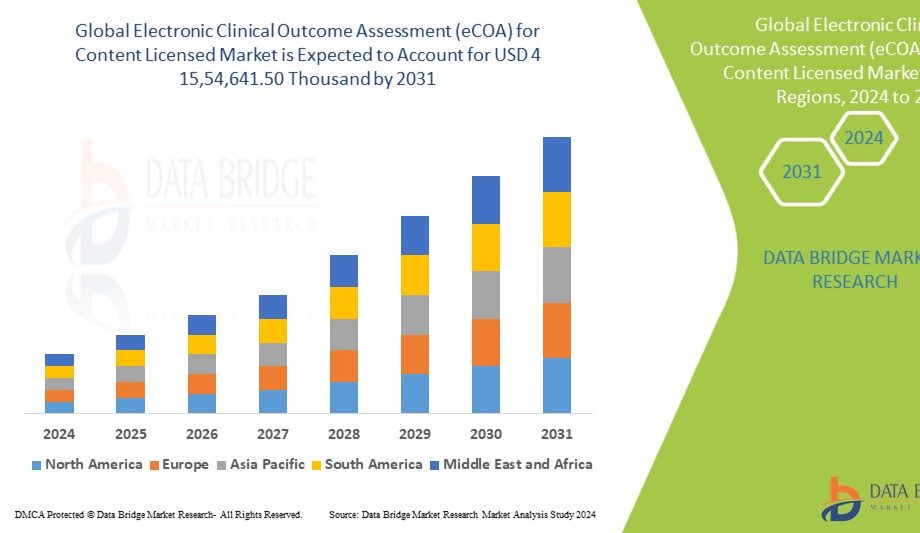
Market Size and Growth Projections
The global Electronic Clinical Outcome Assessment (eCOA) Market was valued at USD 1.7 billion in 2024 and is expected to reach USD 4.3 billion by 2032, expanding at a CAGR of 12.1% during the forecast period. The surge in decentralized clinical trials (DCTs), the need for real-time patient monitoring, and growing regulatory support for electronic data collection are fueling the market’s expansion. Moreover, the COVID-19 pandemic has accelerated the adoption of remote data capture technologies, establishing eCOA as a standard in modern clinical research.
Key Growth Factors
Shift toward decentralized and hybrid clinical trials driving the demand for remote patient data collection.
Regulatory encouragement from agencies such as the FDA and EMA for digital endpoint collection.
Increased use of mobile and wearable technologies for continuous monitoring of patient outcomes.
Improved data accuracy and compliance through automated validation and real-time analytics.
Cost reduction and operational efficiency in clinical data management and trial execution.
Market Segmentation
By Type
Patient-Reported Outcomes (ePRO)
Clinician-Reported Outcomes (eClinRO)
Observer-Reported Outcomes (eObsRO)
Performance Outcomes (PerfO)
By Delivery Mode
Web-Based eCOA Solutions
On-Premises eCOA Platforms
Cloud-Based eCOA Solutions
By End User
Pharmaceutical and Biotechnology Companies
Contract Research Organizations (CROs)
Medical Device Manufacturers
Hospitals and Clinics
Academic and Research Institutions
By Application
Clinical Trials Management
Patient Monitoring and Engagement
Data Integration and Validation
Regulatory Compliance and Reporting
Regional Insights
North America: Holds the largest share due to the presence of major pharmaceutical firms, advanced clinical infrastructure, and strong regulatory backing for digital clinical tools. The U.S. leads in eCOA adoption across both academic and commercial research settings.
Europe: Significant market growth driven by compliance with GDPR standards, high clinical trial activity, and government initiatives supporting digital healthcare.
Asia-Pacific: Expected to witness the fastest growth due to expanding clinical research outsourcing (CRO) industry and increasing investments in healthcare digitalization in India, China, and Japan.
Latin America: Growing participation in global clinical trials and adoption of electronic patient data systems.
Middle East & Africa: Emerging adoption fueled by healthcare modernization and rising partnerships with global pharmaceutical companies.
Key Market Drivers
Increasing complexity of clinical trials requiring precise and real-time data collection.
Growing emphasis on patient-centric data to improve drug efficacy assessments.
Technological advancements in mobile health (mHealth) and wearable devices.
Rising demand for integrated electronic data capture (EDC) and eCOA systems.
Enhanced collaboration between CROs and software vendors for global trial management.
Market Challenges and Restraints
High implementation costs for small and mid-sized sponsors.
Data privacy and security concerns in cloud-based solutions.
Technical challenges in integrating eCOA systems with legacy clinical data management tools.
Limited digital infrastructure in developing regions.
Need for specialized training for investigators and site staff.
Competitive Landscape with Key Companies
The Electronic Clinical Outcome Assessment (eCOA) Market is competitive, with key players focusing on platform integration, real-time analytics, and scalability. Leading vendors are developing unified eClinical solutions combining eCOA with EDC and eConsent platforms.
Major companies include:
IQVIA Inc.
Medidata Solutions (Dassault Systèmes)
Signant Health
Parexel International Corporation
Oracle Corporation
ERT, Inc. (Clario)
Kayentis
YPrime, LLC
CRF Health
ArisGlobal LLC
Castor EDC
Medrio, Inc.
Technological Innovations
AI-powered data validation and analytics improving accuracy and reducing manual review.
Integration of wearables and biosensors for real-time patient outcome tracking.
Voice-based and multilingual patient reporting interfaces enhancing accessibility.
Blockchain technology ensuring transparent and immutable data records.
Cloud-native eCOA platforms for seamless scalability and remote deployment.
SWOT Analysis
Strengths Weaknesses Opportunities Threats
Enhances accuracy and reliability of patient data High initial implementation costs Expansion of decentralized clinical trials Cybersecurity and data breach risks
Supports regulatory compliance and remote monitoring Complexity in system integration Growing CRO outsourcing in emerging markets Resistance to digital adoption in traditional setups
Reduces trial timelines and improves patient engagement Limited awareness in developing countries Integration with wearable and AI technologies Competition from alternative eClinical systems
Future Market Outlook
The Electronic Clinical Outcome Assessment (eCOA) Market is poised for robust growth as clinical research becomes increasingly data-driven and patient-focused. Future advancements will center around AI-integrated platforms, interoperable clinical data ecosystems, and next-generation wearables enabling continuous health monitoring. As regulatory authorities encourage digital transformation in trials, eCOA systems will become indispensable for ensuring data accuracy, patient engagement, and global trial efficiency.
Conclusion
The Electronic Clinical Outcome Assessment (eCOA) Market is redefining how patient outcomes are measured and managed in clinical research. With its ability to capture real-time, high-quality data and streamline trial workflows, eCOA technology enhances clinical accuracy and patient engagement. As the industry shifts toward decentralized and hybrid models, eCOA solutions will remain central to driving transparency, compliance, and efficiency in the global clinical trial ecosystem.

 :
: